CTAC Innovations
The CTAC Site Maturity Assessment Model
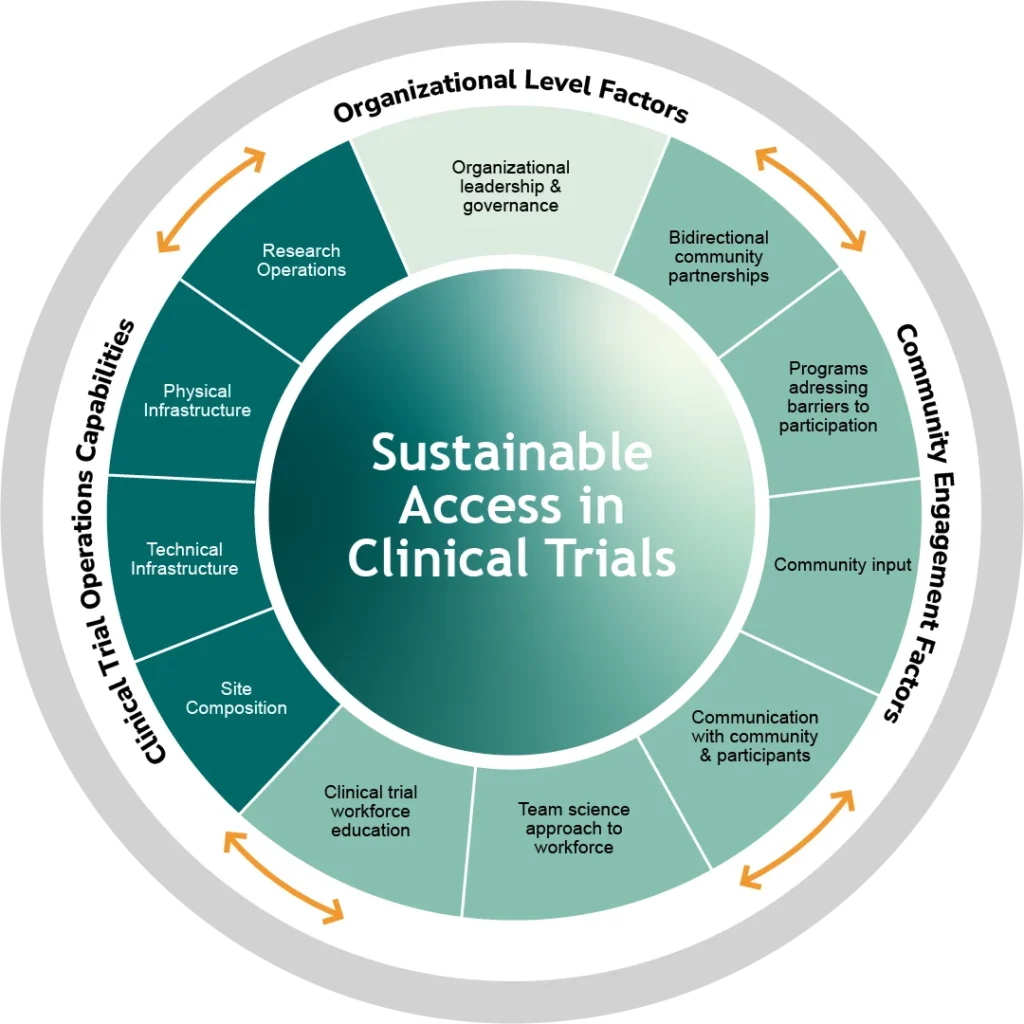
The CTAC Site Maturity Assessment Model is a holistic, collaborative, site-driven, and formative assessment carried out with potential sites to catalogue their current capabilities and identify opportunities for growth in conducting industry-sponsored clinical trials and expanding access to those trials. It is not intended to be evaluative in nature, or to be used to compare sites in the CTAC program or otherwise benchmark against others.
The completed assessment will:
- Inform the site-specific roadmap for capability building during the Learning Phase (with the support of infrastructure partners)
- Serve as a baseline for sites to track progress toward their maturity goals
- Create visibility into site capabilities to help trial sponsors assess interest in placing protocols at the site
Importantly, this model draws from and synthesizes prior initiatives including those led by the Yale Center for Clinical Investigation (YCCI), The Clinical Trials Transformation Initiative (CTTI), The National Academy of Medicine, and Multi-Regional Clinical Trials Centers of Brigham and Women’s Hospital and Harvard (MRCT Center).
Access the Model Here
Citation:
Harris, T., Nunez-Smith, M., Suttiratana, S.C. et al. Supporting diversity in clinical trials: the equitable breakthroughs in medicine site maturity model. Trials 25, 764 (2024). https://doi.org/10.1186/s13063-024-08594-9
The Sponsor Front Door
To ensure sponsors know that non-traditional sites are ready for clinical trials, it’s important to confirm that the site has enough space and resources to conduct high-quality research. The “Sponsor Front Door” helps with this and more.
This online platform provides key details about a site, including facility and leadership videos, areas of medical focus, operational abilities, patient demographics, provider profiles, training, and past audit results. The goal is to make the process easier for both sponsors and sites while improving how clinical trials are matched and set up.